Replacing Hearts.
Restoring Lives.
HEART FAILURE
Heart Failure (HF) is a devastating disease that affects more than 11 million people in the US and Europe, with an incidence of 1.1 million new cases per year. Furthermore, current growth rates predict a 25% increase in the incidence of HF by 2030.
According to the US National Institutes of Health (NIH), 100,000 patients could immediately benefit from a ventricular assist device (VAD) or total artificial heart (TAH), and the European market is similarly sized. Without intervention, patients with severe HF have a bleak outlook. For these patients, drug therapy is a limited, relatively ineffective option. Although a heart transplant would meet their needs, only 4000 donor hearts are available globally each year.
Total Artificial Heart Therapy
Implantation of a TAH is a treatment option for patients with end-stage HF who need support while on a heart transplant waiting list or who do not qualify for a transplant. Removal of the native ventricles allows the device to completely replace the function of the native heart.
DEVICE
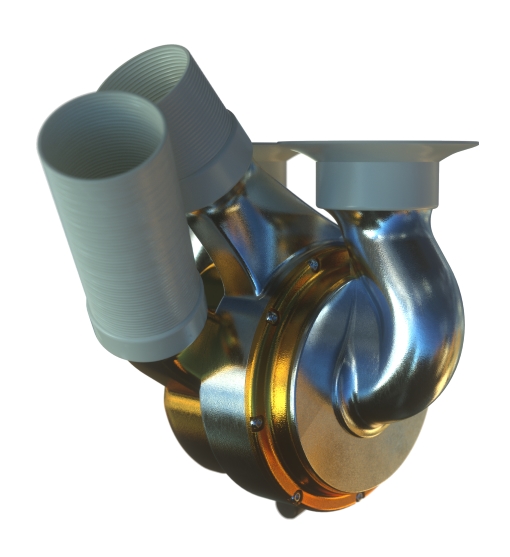
The BiVACOR Total Artificial Heart (TAH) is designed to take over the complete function of a patient’s failing heart.
Powerful
The centrifugal pumps can provide high flows over 12 L/min for dynamic activity.
Smart
Smart controllers adapt the pump operation to changes in the patient’s activity.
Durable
An anticipated device life of up to 10 years or more.
Small
Small enough for a child, powerful enough for an adult.
Portable
A small external controller and batteries to give patients freedom.
TECHNOLOGY
A Single Moving Part
Two centrifugal impellers placed on a single rotor provide perfusion to the left and right sides of the body.
Advantages of a Rotary Pump
Specially designed pump blades allow high flows and low power consumption.
Physiologic Interaction
The device’s patented left-right flow-balancing system allows dynamic adaptation to change in the patient’s physiology.
Active Magnetic Levitation
Magnetic levitation provides precise, stable operation with no mechanical wear.
Blood Compatibility
Special large gaps within the pump reduce blood cell damage and the risk of clotting.
BiVACOR TAH ANIMATION
The BiVACOR TAH is designed to be a long-term device that can replace the total function of the patient’s native heart. The small, compact device uses proven rotary blood pump technology to provide the required cardiac output.
The BiVACOR system comprises a magnetically levitated rotor located between opposing pump casings. The key feature that enables this device to support both the left and right sides of the heart is the left and right impeller blades, which are mounted on either side of the rotating hub. The hub is levitated and rotated via an electromagnetic motor and bearing arrangement on top of the pump casings. The dedicated hydraulic design of the impellers, combined with state-of-the-art magnetic levitation (MAGLEV) technology, permits control of the circulation to be fine-tuned by means of a differential fluid output.
An external controller and batteries provide power to the internal device via a percutaneous driveline.
ABOUT US

BiVACOR® is a privately held company founded in 2008. Our staff includes world-class engineers, medical specialists, and business executives, who are diligently working to advance this ground-breaking technology. Together, we have established a strong collaborative network that extends both nationally and internationally. Our world headquarters is in Huntington Beach, California, with our international office in Brisbane, Australia.
MEET THE TEAM
We are all around the world!

Jim Dillon
Chief Executive Officer
Jim Dillon is an experienced medical device executive whose career focus in heart failure has included developing cardiovascular therapies to enable native heart recovery, reducing the size of a patient’s myocardial infarction, repairing adult and congenital cardiac defects, and volume management for acutely decompensated congestive heart failure patients.
Over his multi-decade career, Mr. Dillon has held a series of corporate strategic and sales leadership positions as a key contributor to multiple successful medical device companies. Prior to his recent role as CEO of clinical-stage private company BioVentrix, he founded and secured financing for Reprieve Cardiovascular, he held senior executive sales and marketing positions with Abiomed and St. Jude Medical.

Daniel Timms, PhD
Chief Technical Officer
Daniel Timms, PhD, is the founder of BiVACOR, Inc. A graduate of the Queensland University of Technology, Dr. Timms completed his postdoctoral training at The Prince Charles Hospital’s cardiac transplant center in Brisbane, Australia, and at the Helmholtz Institute in Aachen, Germany, one of Europe’s leading cardiovascular device research centers.
He has more than 15 years of experience in mechanical circulatory support development.

William Cohn, MD
Chief Medical Officer
William E. Cohn, MD, is Director of the Center for Technology and Innovation; Associate Director of Laboratory Surgery Research in the Center for Cardiac Support; and Co-Director of the Cardiovascular Surgical Research Laboratory at the Texas Heart Institute, in Houston, Texas, USA.
He is a professor of surgery at Baylor College of Medicine in the Division of Transplant and Assist Devices and an adjunct professor of bioengineering at both Rice University and the University of Houston.
His major research interests are focused on the development of medical technology and integration of new technology into clinical practice.
CAREERS
BiVACOR is always accepting applications for motivated engineers and scientists.
Please send us your application documents via the career page or at HR@BiVACOR.com